0 引言
1 深海橄榄岩蛇纹石化过程及研究意义
2 氧同位素测温法的基本原理和技术
3 蛇纹石化过程中蛇纹石氧同位素组成的影响因素
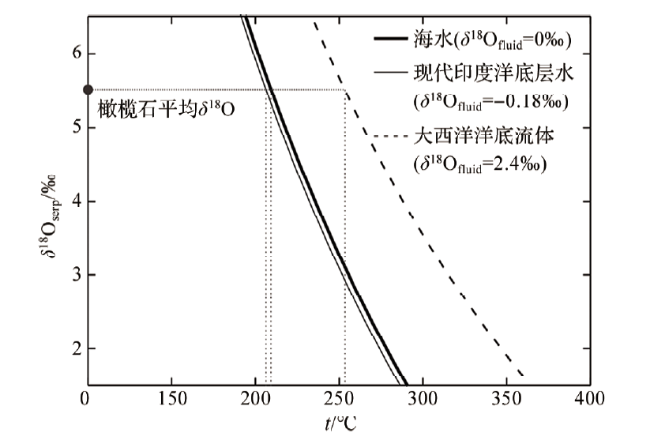
3.1 反应温度和流体氧同位素组成对蛇纹石氧同位素组成的影响
图1 蛇纹石δ18O值随反应温度变化曲线(反应曲线为不同流体端元的模拟结果,其中大西洋洋底流体为大西洋中脊23°N Kane地区的热液流体[48]。分馏公式来自SACCOCIA等[12] 。) Fig.1 The change curve of δ18O value of serpentine with temperature (The curve is generated based on different fluid endmembers, in which the Atlantic Ocean fluid is from the Kane area at the Mid-Atlantic Ridge 23°N[48]. The fractionation formula is from SACCOCIA et al[12].) |
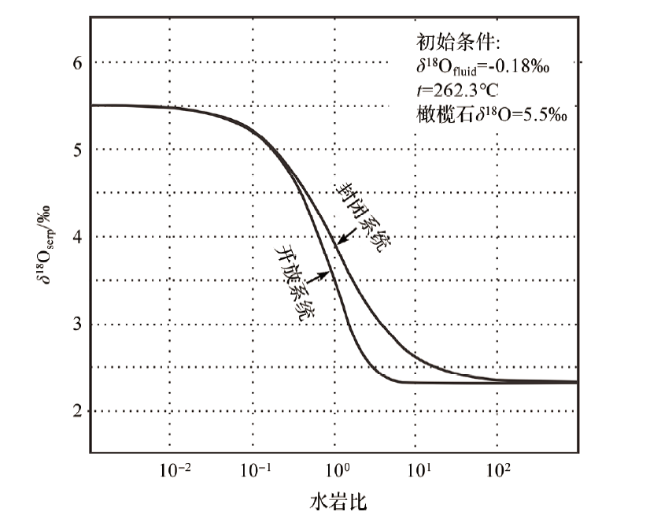
3.2 水岩比对蛇纹石氧同位素组成的影响
3.3 系统封闭条件对蛇纹石氧同位素组成的影响
4 氧同位素在橄榄岩蛇纹石化过程研究中的应用
表1 蛇纹石化橄榄岩次生矿物氧同位素比值和形成温度Tab.1 Oxygen isotope ratios of the secondary minerals in serpentinized peridotites and their formation temperatures |
| 矿物 种类 | δ18O/‰ | 形成温度 | |
|---|---|---|---|
| 温度范围/℃ | 参考文献 | ||
| 蛇纹石 | 1.9~9.6 (106) | 300~320 (利蛇纹石) 320~550 (叶蛇纹石) | 文献[23,36] |
| 磁铁矿 | -10.2~4.75 (92) | 400~450 | 文献[44,11] |
| 滑石 | 3.0~4.7 (11) | 272~323 | 文献[62] |
| 透闪石 | 5.2~8.0 (8) | 350~650 | 文献[23,63-67] |
| 方解石 | 12.67~34.1 (103) | 2~134 | 文献[68] |
| 绿泥石 | 1.12~11.7 (24) | 169~207 | 文献[62] |
注:表格中括号内数字代表参考样品的数目,氧同位素比值数据来自PetDB。 |