Journal of Marine Sciences ›› 2023, Vol. 41 ›› Issue (3): 92-100.DOI: 10.3969/j.issn.1001-909X.2023.03.009
Previous Articles Next Articles
Preparation of standard reference material for reactive phosphorus with seawater matrix
ZHANG Chuan1,2( ), YU Tao1,2, YU Xiaoyan1,2, ZHU Yong3,4, WANG Lifang5, ZHANG Xiaohui1,2
), YU Tao1,2, YU Xiaoyan1,2, ZHU Yong3,4, WANG Lifang5, ZHANG Xiaohui1,2
- 1. National Center of Ocean Standards and Metrology, Tianjin 300112, China
2. Technology Innovation Center for Marine Metrology and Instruments Test, MNR, Tianjin 300112, China
3. Second Institute of Oceanography, MNR, Hangzhou 310012, China
4. Key Laboratory of Marine Ecosystem Dynamics, MNR, Hangzhou 310012, China
5. State Key Laboratory of Marine Environmental Science, Xiamen University, Xiamen 361102, China
-
Received:2023-01-19Revised:2023-03-18Online:2023-09-15Published:2023-10-24
CLC Number:
Cite this article
ZHANG Chuan, YU Tao, YU Xiaoyan, ZHU Yong, WANG Lifang, ZHANG Xiaohui. Preparation of standard reference material for reactive phosphorus with seawater matrix[J]. Journal of Marine Sciences, 2023, 41(3): 92-100.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: http://hyxyj.sio.org.cn/EN/10.3969/j.issn.1001-909X.2023.03.009

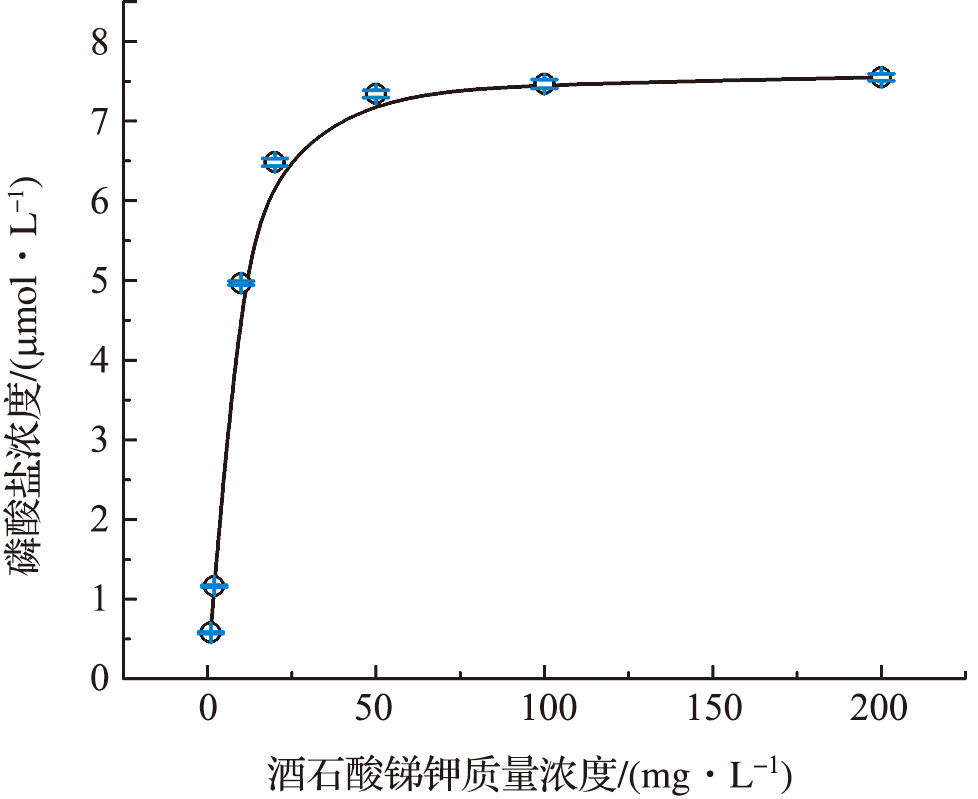
Fig.1 The results of optimization of ascorbic acid mass concentration (The error bar on the dot represents the standard deviation of 3 measurement results. The following pictures are the same.)
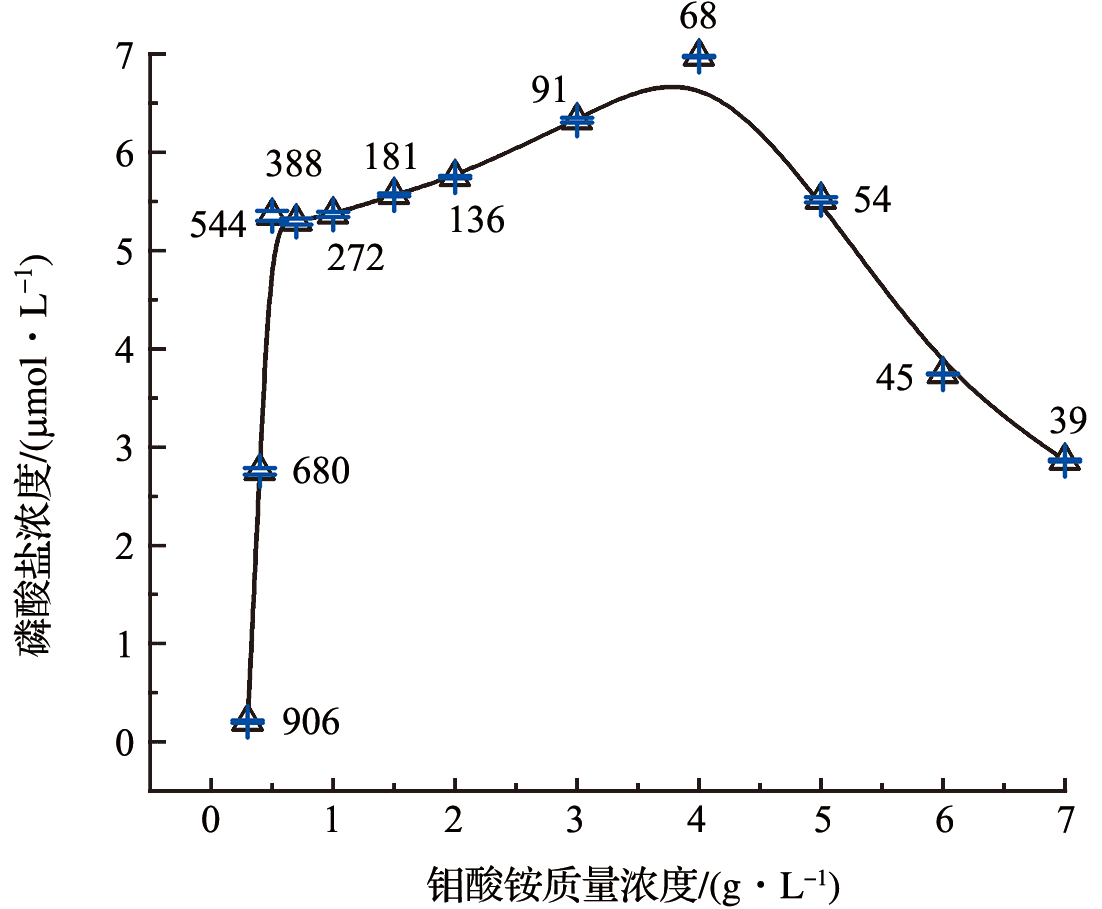
Fig.2 The results of optimization of ammonium molybdate mass concentration (The numbers near the data point represent the ration of [H+] to [MoO24-].)
| CRM | 批次 | CRM特性值 /(μmol·L-1) | 测量值 /(μmol·L-1) | 误差 /(μmol·L-1) |
|---|---|---|---|---|
| NMIJ7603-a | No.HO135 | 3.10±0.06 | 3.15 | +0.05 |
| NMIJ7602-a | No.MO182 | 1.09±0.06 | 1.12 | +0.03 |
Tab.1 The verification results of characterization method accuracy and reliability
| CRM | 批次 | CRM特性值 /(μmol·L-1) | 测量值 /(μmol·L-1) | 误差 /(μmol·L-1) |
|---|---|---|---|---|
| NMIJ7603-a | No.HO135 | 3.10±0.06 | 3.15 | +0.05 |
| NMIJ7602-a | No.MO182 | 1.09±0.06 | 1.12 | +0.03 |
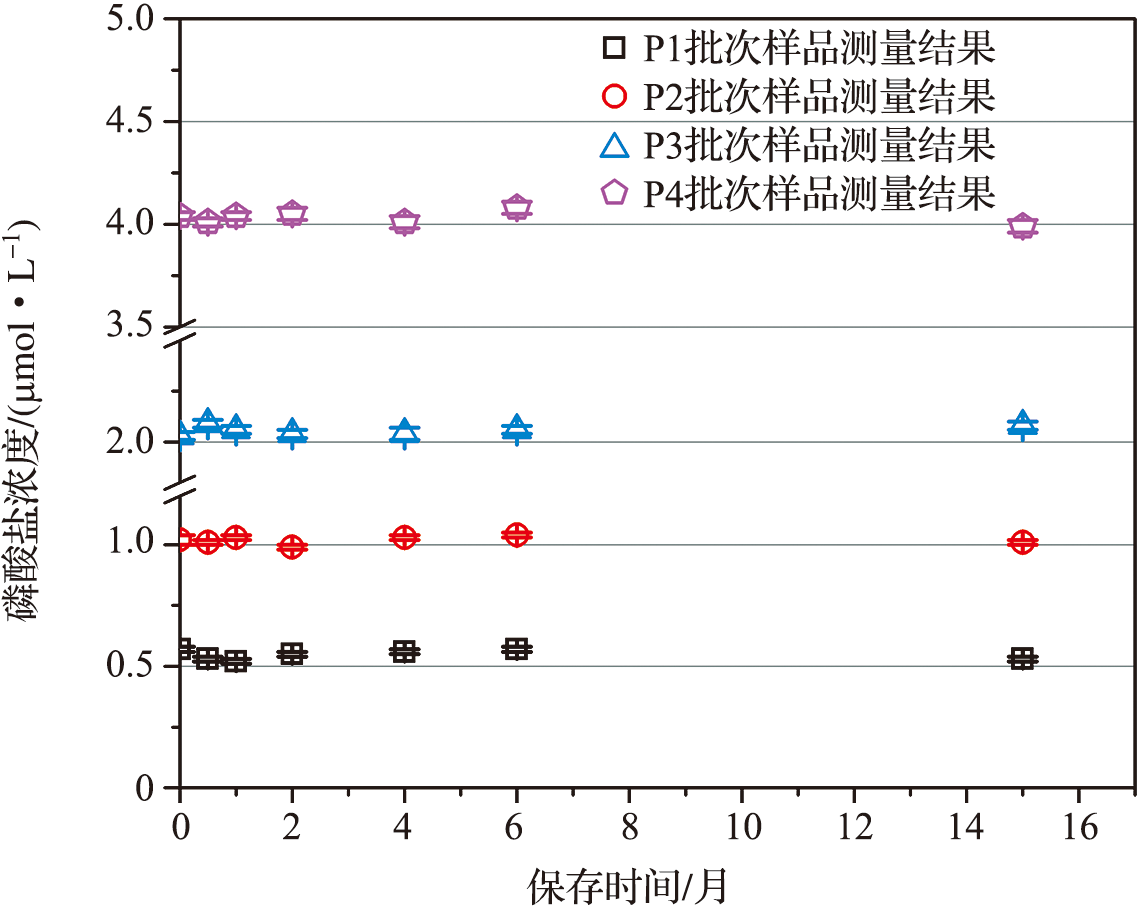
| 序号 | 样品活性磷酸盐浓度测量结果/(μmol·L-1) | |||
|---|---|---|---|---|
| P1批次 | P2批次 | P3批次 | P4批次 | |
| 1 | 0.57 | 1.00 | 2.06 | 4.03 |
| 2 | 0.56 | 1.00 | 2.08 | 4.04 |
| 3 | 0.57 | 1.01 | 2.07 | 4.02 |
| 4 | 0.60 | 1.01 | 2.03 | 4.04 |
| 5 | 0.57 | 1.02 | 2.02 | 4.03 |
| 6 | 0.57 | 1.02 | 1.99 | 4.06 |
| 7 | 0.60 | 1.04 | 2.05 | 4.02 |
| 8 | 0.58 | 1.00 | 2.04 | 4.01 |
| 9 | 0.57 | 1.04 | 2.11 | 4.04 |
| 平均值 | 0.58 | 1.02 | 2.05 | 4.03 |
| RSD/% | 2.45 | 1.57 | 1.72 | 0.37 |
Tab.2 The verification results of characterization method precision
| 序号 | 样品活性磷酸盐浓度测量结果/(μmol·L-1) | |||
|---|---|---|---|---|
| P1批次 | P2批次 | P3批次 | P4批次 | |
| 1 | 0.57 | 1.00 | 2.06 | 4.03 |
| 2 | 0.56 | 1.00 | 2.08 | 4.04 |
| 3 | 0.57 | 1.01 | 2.07 | 4.02 |
| 4 | 0.60 | 1.01 | 2.03 | 4.04 |
| 5 | 0.57 | 1.02 | 2.02 | 4.03 |
| 6 | 0.57 | 1.02 | 1.99 | 4.06 |
| 7 | 0.60 | 1.04 | 2.05 | 4.02 |
| 8 | 0.58 | 1.00 | 2.04 | 4.01 |
| 9 | 0.57 | 1.04 | 2.11 | 4.04 |
| 平均值 | 0.58 | 1.02 | 2.05 | 4.03 |
| RSD/% | 2.45 | 1.57 | 1.72 | 0.37 |
| 批次 | 实测活性磷酸盐浓度 平均值/(μmol·L-1) | RSD/% | F值 |
|---|---|---|---|
| P1 | 0.57 | 1.89 | 1.37 |
| P2 | 1.02 | 1.34 | 2.20 |
| P3 | 2.05 | 0.91 | 2.01 |
| P4 | 4.02 | 0.62 | 2.20 |
Tab.3 The results of homogeneity test
| 批次 | 实测活性磷酸盐浓度 平均值/(μmol·L-1) | RSD/% | F值 |
|---|---|---|---|
| P1 | 0.57 | 1.89 | 1.37 |
| P2 | 1.02 | 1.34 | 2.20 |
| P3 | 2.05 | 0.91 | 2.01 |
| P4 | 4.02 | 0.62 | 2.20 |
| 批次 | β1 | s(β1) | t95,n-2×s(β1) |
|---|---|---|---|
| P1 | -1.797 7×10-5 | 5.661 8×10-5 | 1.455 6×10-4 |
| P2 | 4.960 5×10-7 | 4.656 0×10-5 | 1.197 1×10-4 |
| P3 | 5.209 6×10-5 | 5.645 7×10-5 | 1.451 5×10-4 |
| P4 | -7.427 9×10-5 | 7.648 5×10-5 | 1.966 4×10-4 |
Tab.4 The results of long-term stability test
| 批次 | β1 | s(β1) | t95,n-2×s(β1) |
|---|---|---|---|
| P1 | -1.797 7×10-5 | 5.661 8×10-5 | 1.455 6×10-4 |
| P2 | 4.960 5×10-7 | 4.656 0×10-5 | 1.197 1×10-4 |
| P3 | 5.209 6×10-5 | 5.645 7×10-5 | 1.451 5×10-4 |
| P4 | -7.427 9×10-5 | 7.648 5×10-5 | 1.966 4×10-4 |
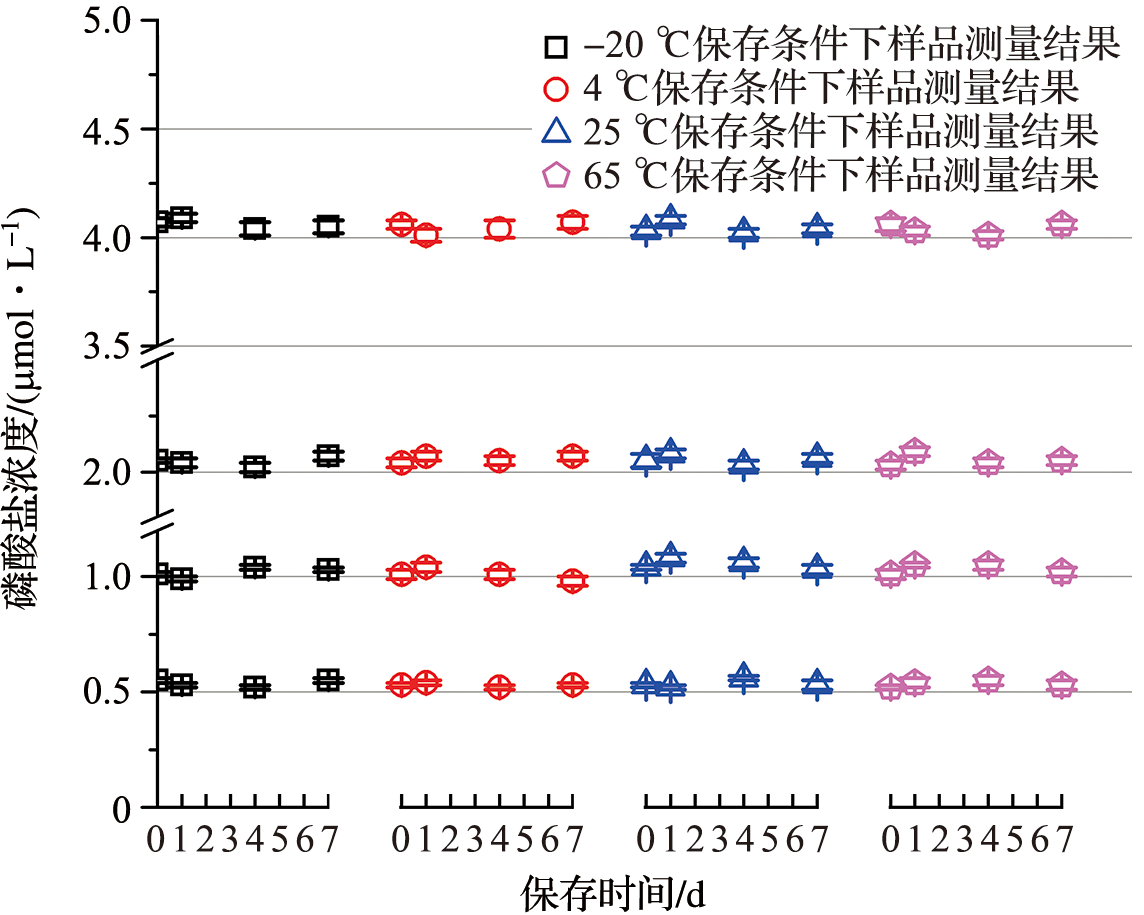
| 环境 温度/℃ | 批次 | β1 | s(β1) | t95,n-2×s(β1) |
|---|---|---|---|---|
| -20 | P1 | -3.333 3×10-4 | 2.255 7×10-3 | 9.706 5×10-3 |
| P2 | 5.000 0×10-3 | 2.343 6×10-3 | 1.008 4×10-2 | |
| P3 | 2.333 3×10-3 | 2.934 5×10-3 | 1.262 7×10-2 | |
| P4 | -5.000 0×10-3 | 2.345 9×10-3 | 1.008 4×10-2 | |
| 4 | P1 | -2.333 3×10-3 | 1.536 6×10-3 | 6.611 9×10-3 |
| P2 | -6.000 0×10-3 | 2.335 5×10-3 | 1.005 0×10-2 | |
| P3 | 2.333 3×10-3 | 1.968 8×10-3 | 8.471 8×10-3 | |
| P4 | 4.000 0×10-3 | 3.503 2×10-3 | 1.507 4×10-2 | |
| 25 | P1 | -1.666 7×10-3 | 2.487 3×10-3 | 1.070 3×10-2 |
| P2 | -3.333 3×10-3 | 2.940 9×10-3 | 1.265 5×10-2 | |
| P3 | -1.333 3×10-3 | 3.073 2×10-3 | 1.322 4×10-2 | |
| P4 | -2.333 3×10-3 | 3.805 6×10-3 | 1.637 5×10-2 | |
| 65 | P1 | -1.000 0×10-3 | 1.887 0×10-3 | 8.119 6×10-3 |
| P2 | 0 | 3.107 9×10-3 | 1.337 3×10-2 | |
| P3 | -1.000 0×10-3 | 3.936 0×10-3 | 1.693 7×10-2 | |
| P4 | -3.333 3×10-4 | 3.689 3×10-3 | 1.587 5×10-2 |
Tab.5 The results of short-term stability test
| 环境 温度/℃ | 批次 | β1 | s(β1) | t95,n-2×s(β1) |
|---|---|---|---|---|
| -20 | P1 | -3.333 3×10-4 | 2.255 7×10-3 | 9.706 5×10-3 |
| P2 | 5.000 0×10-3 | 2.343 6×10-3 | 1.008 4×10-2 | |
| P3 | 2.333 3×10-3 | 2.934 5×10-3 | 1.262 7×10-2 | |
| P4 | -5.000 0×10-3 | 2.345 9×10-3 | 1.008 4×10-2 | |
| 4 | P1 | -2.333 3×10-3 | 1.536 6×10-3 | 6.611 9×10-3 |
| P2 | -6.000 0×10-3 | 2.335 5×10-3 | 1.005 0×10-2 | |
| P3 | 2.333 3×10-3 | 1.968 8×10-3 | 8.471 8×10-3 | |
| P4 | 4.000 0×10-3 | 3.503 2×10-3 | 1.507 4×10-2 | |
| 25 | P1 | -1.666 7×10-3 | 2.487 3×10-3 | 1.070 3×10-2 |
| P2 | -3.333 3×10-3 | 2.940 9×10-3 | 1.265 5×10-2 | |
| P3 | -1.333 3×10-3 | 3.073 2×10-3 | 1.322 4×10-2 | |
| P4 | -2.333 3×10-3 | 3.805 6×10-3 | 1.637 5×10-2 | |
| 65 | P1 | -1.000 0×10-3 | 1.887 0×10-3 | 8.119 6×10-3 |
| P2 | 0 | 3.107 9×10-3 | 1.337 3×10-2 | |
| P3 | -1.000 0×10-3 | 3.936 0×10-3 | 1.693 7×10-2 | |
| P4 | -3.333 3×10-4 | 3.689 3×10-3 | 1.587 5×10-2 |
| 批次 | 格拉布 斯检验 | 科克伦 检验 | 狄克逊 检验 | 正态分 布检验 | 总体平均值 /(μmol·L-1) | 总体SD /(μmol·L-1) |
|---|---|---|---|---|---|---|
| P1 | 通过 | 等精度 | 无离群值 | 通过 | 0.53 | 0.03 |
| P2 | 通过 | 等精度 | 无离群值 | 通过 | 1.02 | 0.02 |
| P3 | 通过 | 等精度 | 无离群值 | 通过 | 2.03 | 0.04 |
| P4 | 通过 | 等精度 | 无离群值 | 通过 | 4.01 | 0.04 |
Tab.6 The results of raw data statistical test
| 批次 | 格拉布 斯检验 | 科克伦 检验 | 狄克逊 检验 | 正态分 布检验 | 总体平均值 /(μmol·L-1) | 总体SD /(μmol·L-1) |
|---|---|---|---|---|---|---|
| P1 | 通过 | 等精度 | 无离群值 | 通过 | 0.53 | 0.03 |
| P2 | 通过 | 等精度 | 无离群值 | 通过 | 1.02 | 0.02 |
| P3 | 通过 | 等精度 | 无离群值 | 通过 | 2.03 | 0.04 |
| P4 | 通过 | 等精度 | 无离群值 | 通过 | 4.01 | 0.04 |
| 实验室 代号 | P1批次 | P2批次 | P3批次 | P4批次 | ||||
|---|---|---|---|---|---|---|---|---|
| 平均值 | SD | 平均值 | SD | 平均值 | SD | 平均值 | SD | |
| A | 0.56 | 0.005 | 1.02 | 0.014 | 2.03 | 0.008 | 4.03 | 0.015 |
| B | 0.49 | 0.008 | 0.98 | 0.008 | 1.98 | 0.008 | 3.96 | 0.019 |
| C | 0.52 | 0.013 | 1.03 | 0.015 | 2.08 | 0.010 | 4.06 | 0.010 |
| D | 0.56 | 0.000 | 1.05 | 0.004 | 2.08 | 0.010 | 4.00 | 0.008 |
| E | 0.50 | 0.009 | 1.02 | 0.009 | 2.00 | 0.008 | 3.98 | 0.027 |
| F | 0.56 | 0.008 | 1.01 | 0.009 | 2.04 | 0.018 | 4.04 | 0.014 |
Tab.7
| 实验室 代号 | P1批次 | P2批次 | P3批次 | P4批次 | ||||
|---|---|---|---|---|---|---|---|---|
| 平均值 | SD | 平均值 | SD | 平均值 | SD | 平均值 | SD | |
| A | 0.56 | 0.005 | 1.02 | 0.014 | 2.03 | 0.008 | 4.03 | 0.015 |
| B | 0.49 | 0.008 | 0.98 | 0.008 | 1.98 | 0.008 | 3.96 | 0.019 |
| C | 0.52 | 0.013 | 1.03 | 0.015 | 2.08 | 0.010 | 4.06 | 0.010 |
| D | 0.56 | 0.000 | 1.05 | 0.004 | 2.08 | 0.010 | 4.00 | 0.008 |
| E | 0.50 | 0.009 | 1.02 | 0.009 | 2.00 | 0.008 | 3.98 | 0.027 |
| F | 0.56 | 0.008 | 1.01 | 0.009 | 2.04 | 0.018 | 4.04 | 0.014 |
| 项目 | P1批次 | P2批次 | P3批次 | P4批次 |
|---|---|---|---|---|
| ubb | 0.006 | 0.011 | 0.014 | 0.019 |
| ults | 0.021 | 0.017 | 0.021 | 0.028 |
| uchar | 0.014 | 0.010 | 0.017 | 0.015 |
| uCRM | 0.026 | 0.023 | 0.030 | 0.037 |
| U(k=2) | 0.052 | 0.046 | 0.060 | 0.074 |
| Urel(k=2) | 10% | 5% | 3% | 2% |
Tab.8
| 项目 | P1批次 | P2批次 | P3批次 | P4批次 |
|---|---|---|---|---|
| ubb | 0.006 | 0.011 | 0.014 | 0.019 |
| ults | 0.021 | 0.017 | 0.021 | 0.028 |
| uchar | 0.014 | 0.010 | 0.017 | 0.015 |
| uCRM | 0.026 | 0.023 | 0.030 | 0.037 |
| U(k=2) | 0.052 | 0.046 | 0.060 | 0.074 |
| Urel(k=2) | 10% | 5% | 3% | 2% |
| [1] |
HOWARTH R W. Nutrient limitation of net primary production in marine ecosystems[J]. Annual Review of Ecology and Systematics, 1988, 19: 89-110.
DOI URL |
| [2] |
LE MOAL M, GASCUEL-ODOUX C, MÉNESGUEN A, et al. Eutrophication: A new wine in an old bottle[J]. Science of the Total Environment, 2019, 651: 1-11.
DOI URL |
| [3] |
BENNETT E M, CARPENTER S R, CARACO N F. Human impact on erodable phosphorus and eutrophication: A global perspective[J]. BioScience, 2001, 51(3): 227.
DOI URL |
| [4] |
MAHER W, WOO L. Procedures for the storage and digestion of natural waters for the determination of filterable reactive phosphorus, total filterable phosphorus and total phosphorus[J]. Analytica Chimica Acta, 1998, 375(1/2): 5-47.
DOI URL |
| [5] |
ROBARDS K, MCKELVIE I D, BENSON R L, et al. Determination of carbon, phosphorus, nitrogen and silicon species in waters[J]. Analytica Chimica Acta, 1994, 287(3): 147-190.
DOI URL |
| [6] |
JARVIE H P, WITHERS J A, NEAL C. Review of robust measurement of phosphorus in river water: Sampling, storage, fractionation and sensitivity[J]. Hydrology and Earth System Sciences, 2002, 6(1): 113-131.
DOI URL |
| [7] | 国家质量监督检验检疫总局, 国家标准化管理委员会. 海洋调查规范第4部分:海水化学要素调查:GB/T 12763.4—2007[S]. 北京: 中国标准出版社, 2007. |
| General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization administration. Specifications for oceanographic survey—Part 4: Survey of chemical parameters in sea water: GB/T 12763.4—2007[S]. Beijing: Standards Press of China, 2007. | |
| [8] | 国家质量监督检验检疫总局, 国家标准化管理委员会. 海洋监测规范第4部分:海水分析: GB 17378.4—2007[S]. 中国标准出版社, 2008. |
| General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization administration. The specification for marine monitoring—Part 4: Seawater analysis: GB 17378.4—2007[S]. Beijing: Standards Press of China, 2008. | |
| [9] |
王丽芳, 黄韬, 杜川军, 等. 不同海水营养盐现场连续观测系统的比较研究[J]. 热带海洋学报, 2021, 40(3):103-113.
DOI |
| WANG L F, HUANG T, DU C J, et al. Comparison of different continuous in situ observation systems in seawater[J]. Journal of Tropical Oceanography, 2021, 40(3):103-113. | |
| [10] |
CHOQUETTE S J, DUEWER D L, SHARPLESS K E. NIST reference materials: Utility and future[J]. Annual Review of Analytical Chemistry, 2020, 13: 453-474.
DOI URL |
| [11] | 卢晓华, 薄梦, 吴雪, 等. 标准物质领域发展现状及趋势[J]. 化学试剂, 2022, 44(10):1403-1410. |
| LU X H, BO M, WU X, et al. Current situation and trends on the development of reference materials[J]. Chemical Reagents, 2022, 44(10): 1403-1410. | |
| [12] | 朱勇, 施晓来, 刘强, 等. 海水营养盐标准物质的研制和发展[J]. 海洋开发与管理, 2018, 35(6):30-33. |
| ZHU Y, SHI X L, LIU Q, et al. Current status and development of a certified reference material for nutrients in seawater[J]. Ocean Development and Management, 2018, 35(6): 30-33. | |
| [13] | AOYAMA M, ABAD M, LUDWICHOWSKI K U, et al. IOCCP-JAMSTEC 2015 inter-laboratory calibration exercise of a certified reference material for nutrients in seawater[M]. Yokosuka: Japan Agency for Marine-Earth Science and Technology, 2016. |
| [14] | 徐燕青, 高生泉, 陈建芳, 等. 氢氧化镁共沉淀法测定海水中纳摩尔级活性磷酸盐[J]. 分析化学, 2011, 39(1):133-136. |
| XU Y Q, GAO S Q, CHEN J F, et al. Determination of reactive phosphate in nanomolar level in sea water with Mg(OH)2 Co-precipitation[J]. Chinese Journal of Analytical Chemistry, 2011, 39(1): 133-136. | |
| [15] |
MURPHY J, RILEY J P. A modified single solution method for the determination of phosphate in natural waters[J]. Analytica Chimica Acta, 1962, 27: 31-36.
DOI URL |
| [16] |
CHEN Y L L, CHEN H Y. Seasonal dynamics of primary and new production in the northern South China Sea: The significance of river discharge and nutrient advection[J]. Deep Sea Research Part I: Oceanographic Research Papers, 2006, 53(6): 971-986.
DOI URL |
| [17] |
LIANG Y, YUAN D X, LI Q L, et al. Flow injection analysis of nanomolar level orthophosphate in seawater with solid phase enrichment and colorimetric detection[J]. Marine Chemistry, 2007, 103(1/2): 122-130.
DOI URL |
| [18] |
MA J, YUAN D X, LIANG Y. Sequential injection analysis of nanomolar soluble reactive phosphorus in seawater with HLB solid phase extraction[J]. Marine Chemistry, 2008, 111(3/4): 151-159.
DOI URL |
| [19] |
YUAN Y, WANG S, YUAN D X, et al. A simple and cost-effective manual solid phase extraction method for the determination of nanomolar dissolved reactive phosphorus in aqueous samples[J]. Limnology and Oceanography: Methods, 2016, 14(2): 79-86.
DOI URL |
| [20] | 国家市场监督管理总局. 标准物质的定值及均匀性、稳定性评估: JJF 1343—2022[S]. 北京: 中国标准出版社, 2022. |
| State Administration for Market Regulation. Characterization, homogeneity and stability assessment of reference materials: JJF 1343—2022[S]. Beijing: Standards Press of China, 2022. | |
| [21] |
BECKER S, AOYAMA M, WOODWARD E M S, et al. GO-SHIP repeat hydrography nutrient manual: The precise and accurate determination of dissolved inorganic nutrients in seawater, using continuous flow analysis methods[J]. Frontiers in Marine Science, 2020, 7: 581790.
DOI URL |
| [22] |
PAI S C, YANG C C, RILEY J P. Effects of acidity and molybdate concentration on the kinetics of the formation of the phosphoantimonylmolybdenum blue complex[J]. Analytica Chimica Acta, 1990, 229: 115-120.
DOI URL |
| [23] |
ZHANG J Z, FISCHER C J, ORTNER P B. Continuous flow analysis of phosphate in natural waters using hydrazine as a reductant[J]. International Journal of Environmental Analytical Chemistry, 2001, 80(1): 61-73.
DOI URL |
| [24] |
LEVINE H, ROWE J J, GRIMALDI F S. Molybdenum blue reaction and determination of phosphours in waters containing arsenic, silicon, and germanium[J]. Analytical Chemistry, 1955, 27(2): 258-262.
DOI URL |
| [25] |
DRUMMOND L, MAHER W. Determination of phosphorus in aqueous solution via formation of the phosphoantimon-ylmolybdenum blue complex. Re-examination of optimum conditions for the analysis of phosphate[J]. Analytica Chimica Acta, 1995, 302(1): 69-74.
DOI URL |
| [1] | LIU Qiang, SHI Xiaolai, LÜ Haiyan, ZHU Yong, WU Bin. Preparation of standard reference material for heavy metals in low salinity seawater [J]. Journal of Marine Sciences, 2020, 38(3): 76-82. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||