海洋学研究 ›› 2025, Vol. 43 ›› Issue (1): 34-46.DOI: 10.3969/j.issn.1001-909X.2025.01.004
深海多金属结核与周围沉积物中的微生物群落结构特征及其功能
何心怡1,2,3( ), 刘倩2,4, 李小虎1,2,3,*(
), 刘倩2,4, 李小虎1,2,3,*( ), 李正刚2,3, 王浩2,3, 朱志敏2,3, 李怀明2,3
), 李正刚2,3, 王浩2,3, 朱志敏2,3, 李怀明2,3
- 1.海底科学与划界全国重点实验室,上海交通大学 海洋学院,上海 200240
2.海底科学与划界全国重点实验室,自然资源部第二海洋研究所,浙江 杭州 310012
3.自然资源部海底科学重点实验室,浙江 杭州 310012
4.自然资源部海洋生态系统动力学重点实验室,浙江 杭州 310012
-
收稿日期:2024-04-09修回日期:2024-05-24出版日期:2025-03-15发布日期:2025-05-30 -
通讯作者:*李小虎(1979—),男,研究员,主要从事海底资源勘查、评价和成矿理论研究,E-mail: xhli@sio.org.cn。 -
作者简介:何心怡(1998—),女,四川省德阳市人,主要从事多金属结核微生物成矿作用研究,E-mail:xinyihe1129@163.com。 -
基金资助:国家自然科学基金(U2244222);国家重点研发计划(2023YFC2811305)
Microbial community structure and function in deep-sea polymetallic nodules and surrounding sediments
HE Xinyi1,2,3( ), LIU Qian2,4, LI Xiaohu1,2,3,*(
), LIU Qian2,4, LI Xiaohu1,2,3,*( ), LI Zhenggang2,3, WANG Hao2,3, ZHU Zhimin2,3, LI Huaiming2,3
), LI Zhenggang2,3, WANG Hao2,3, ZHU Zhimin2,3, LI Huaiming2,3
- 1. State Key Laboratory of Submarine Geoscience, School of Oceanography, Shanghai Jiao Tong University, Shanghai 200240, China
2. State Key Laboratory of Submarine Geoscience, Second Institute of Oceanography, MNR, Hangzhou 310012, China
3. Key Laboratory of Submarine Geosciences, MNR, Hangzhou 310012, China
4. Key Laboratory of Marine Ecosystem Dynamics, MNR, Hangzhou 310012, China
-
Received:2024-04-09Revised:2024-05-24Online:2025-03-15Published:2025-05-30
摘要:
深海多金属结核和沉积物中赋存丰富的微生物,研究其群落结构特征和功能对认识深海微生物基因资源和微生物成矿作用具有重要的科学意义。目前,对于深海多金属结核分布区的结核内部及结核周围沉积物的细菌群落多样性和结构特征的研究较少,特别是对微生物参与多金属结核成矿的认识十分有限。本研究利用16S rRNA全长测序技术获得了太平洋区域不同类型多金属结核与周围沉积物中的细菌群落组成,通过扫描电镜和能谱分析观察到类细菌微球结构以及结构表面的金属元素分布。研究结果表明:细菌群落组成在不同结核和沉积物中存在差异,变形菌门(Proteobacteria)和拟杆菌门(Bacteroidetes)为优势门类。有些功能类群可能因具备金属氧化还原或生物膜生成能力而参与了多金属结核成矿过程,如能够驱动结核锰元素循环和锰矿物形成的希瓦氏菌属(Shewanella)和科尔韦尔氏菌属(Colwellia)。类细菌微球结构能够促进金属元素在其表面聚集,可能为矿物沉淀提供了位点。研究进一步深化了关于微生物功能及其与矿物相互作用的认识,对于认识深部生命圈的生物地球化学循环和微生物成矿过程具有积极意义。
中图分类号:
引用本文
何心怡, 刘倩, 李小虎, 李正刚, 王浩, 朱志敏, 李怀明. 深海多金属结核与周围沉积物中的微生物群落结构特征及其功能[J]. 海洋学研究, 2025, 43(1): 34-46.
HE Xinyi, LIU Qian, LI Xiaohu, LI Zhenggang, WANG Hao, ZHU Zhimin, LI Huaiming. Microbial community structure and function in deep-sea polymetallic nodules and surrounding sediments[J]. Journal of Marine Sciences, 2025, 43(1): 34-46.
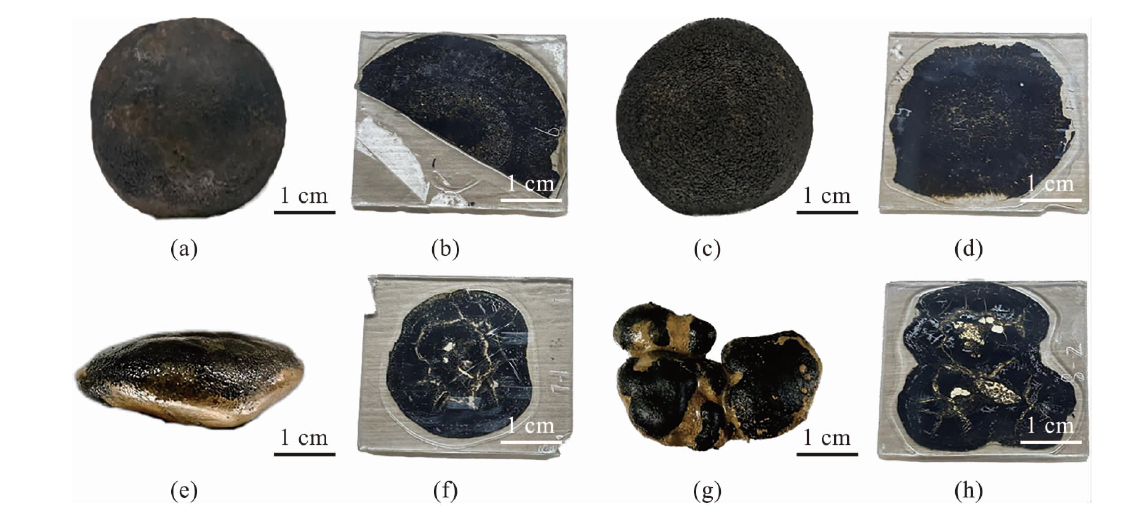
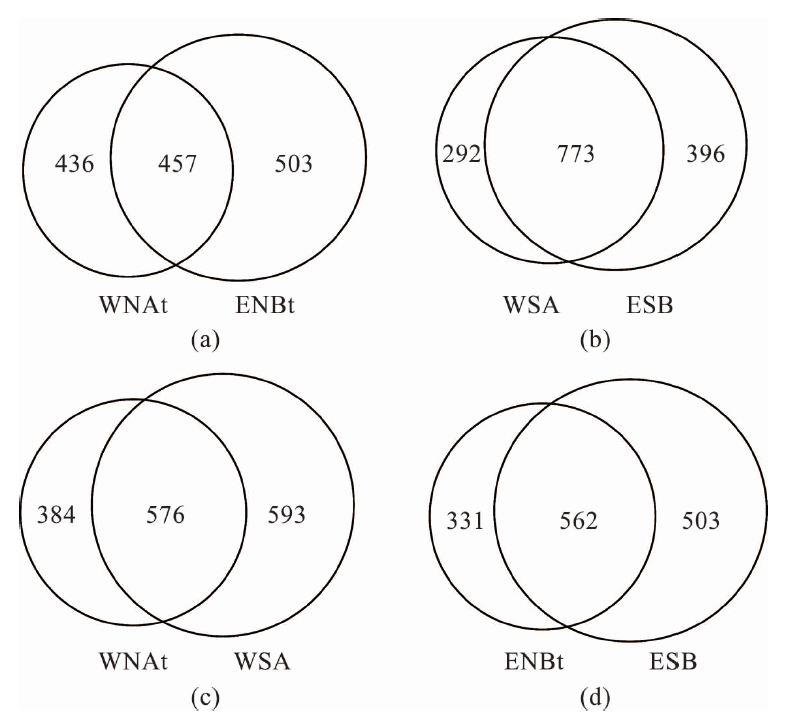
图2 结核样品主要形态与对应薄片的外观 (a~d: 西太平洋M2-BC78站位所采结核样品及其对应的薄片;e~h:东太平洋KW1-BC05站位所采结核样品及其对应的薄片。)
Fig.2 Main morphologies of nodule samples and the appearance of corresponding thin sections (a-d: Morphologies and corresponding thin sections of nodule samples collected at M2-BC78 site from the western Pacific; e-h: Morphologies and corresponding thin sections of nodule samples collected at KW1-BC05 site from the eastern Pacific.)
| 项目 | 西太平洋M2-MC02站 | 东太平洋KW1-MC04站 | |||
|---|---|---|---|---|---|
| 结核 (WNAt) | 沉积物 (WSA) | 结核 (ENBt) | 沉积物 (ESB) | ||
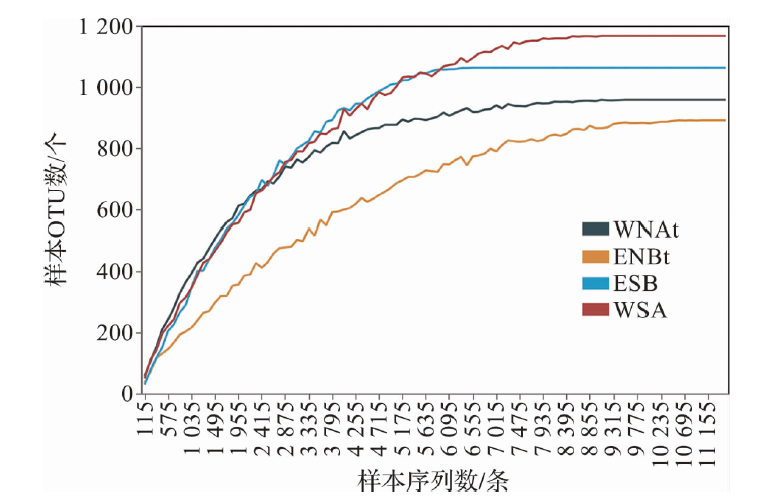
| 序列数/条 | 10 319 | 9 535 | 11 553 | 6 862 | |
| OTU数/个 | 960 | 1 169 | 893 | 1 065 | |
| ACE | 1 024 | 1 490 | 1 201 | 1 512 | |
| Shannon指数 | 8.38 | 8.45 | 6.26 | 7.45 | |
| 覆盖率/% | 99 | 99 | 99 | 99 | |
表1 细菌序列信息与多样性指数
Tab.1 Bacterial sequence information and diversity index
| 项目 | 西太平洋M2-MC02站 | 东太平洋KW1-MC04站 | |||
|---|---|---|---|---|---|
| 结核 (WNAt) | 沉积物 (WSA) | 结核 (ENBt) | 沉积物 (ESB) | ||
| 序列数/条 | 10 319 | 9 535 | 11 553 | 6 862 | |
| OTU数/个 | 960 | 1 169 | 893 | 1 065 | |
| ACE | 1 024 | 1 490 | 1 201 | 1 512 | |
| Shannon指数 | 8.38 | 8.45 | 6.26 | 7.45 | |
| 覆盖率/% | 99 | 99 | 99 | 99 | |
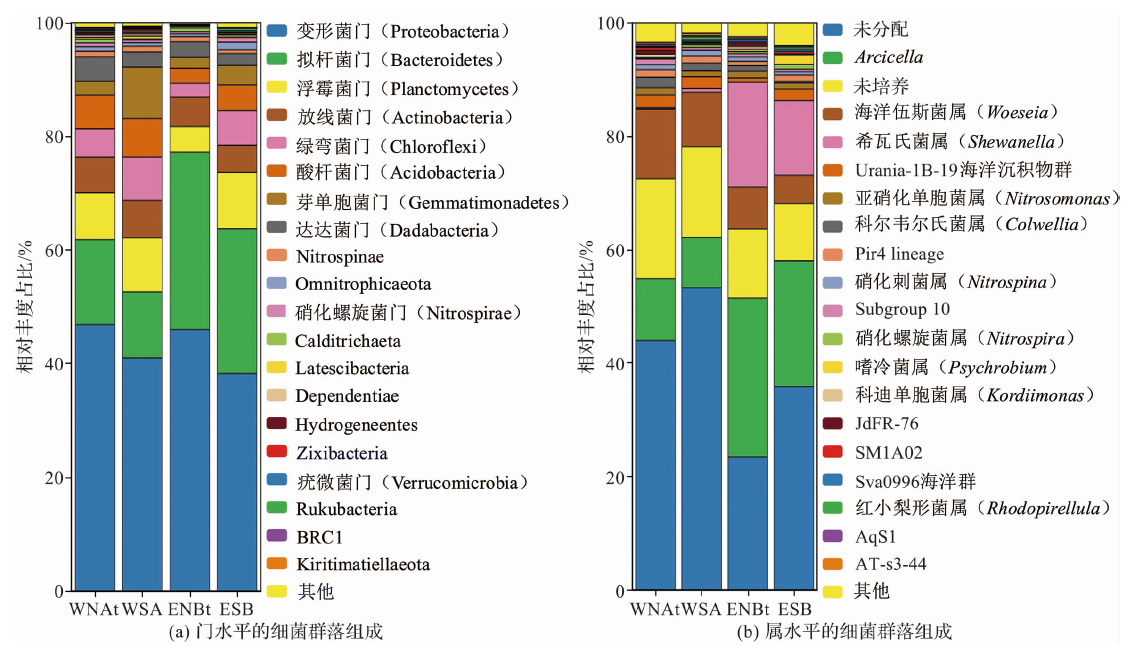
图5 东、西太平洋结核和沉积物中的细菌群落组成 (“未分配”代表无法明确分类到已知属的序列集合,“未培养”代表无法通过传统培养方法从环境中培养的属集合。)
Fig.5 Bacterial community composition in nodules and sediments of the eastern and western Pacific Ocean (“unassigned” represents a collection of sequences that cannot be definitively classified into known genera, “uncultured” represents a collection of genera that cannot be cultivated from the environment using traditional cultivation methods.)
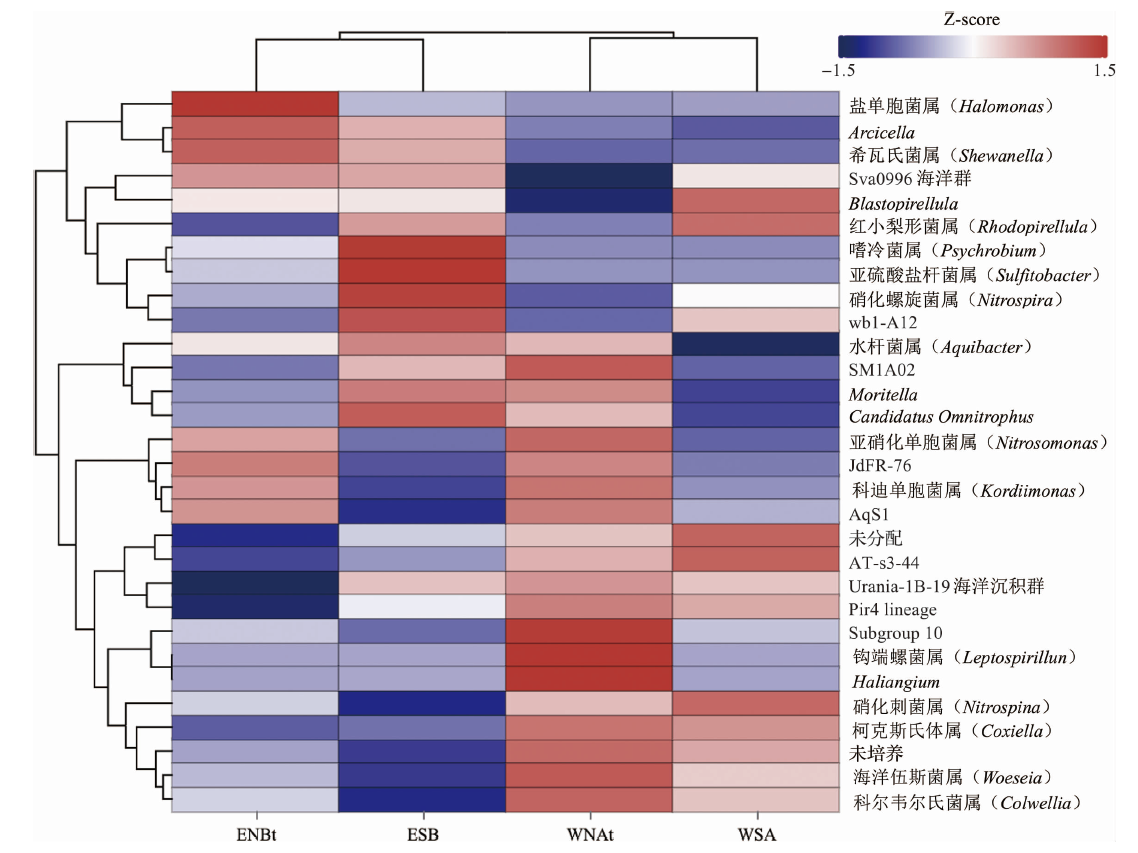
图6 属水平(前30位)物种相对丰度平均聚类热图 (图中颜色代表属在样品中的富集程度,颜色越红表示富集程度越大;“未分配”代表无法明确分类到已知属的序列集合,“未培养”代表无法通过传统培养方法从环境中培养的属集合。)
Fig.6 Relative abundance average clustering heatmap at the genus level (top 30) (The color in the figure signifies the degree of enrichment of the genus within the sample, with deeper red hues indicating a higher level of enrichment. “unassigned” represents a collection of sequences that cannot be definitively classified into known genera, “uncultured” represents a collection of genera that cannot be cultivated from the environment using traditional cultivation methods.)
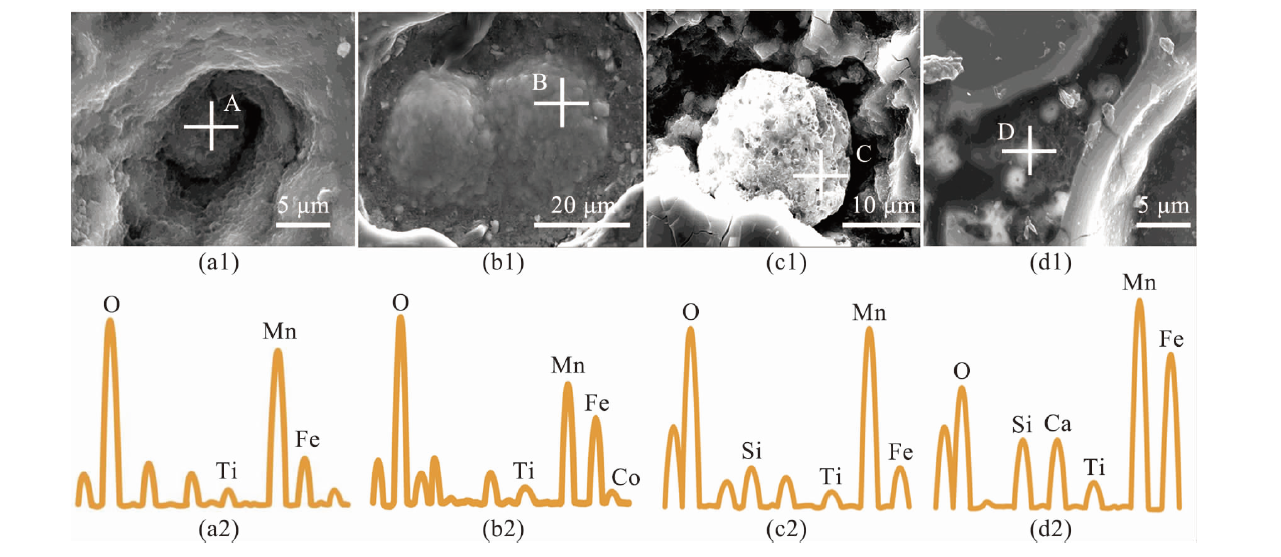
图8 结核内微球结构及对应点位的能量色散光谱(EDS)结果 (a~b:西太平洋结核中的微球结构和EDS结果;c~d:东太平洋结核中的微球结构和EDS结果。)
Fig.8 Microsphere structures in the nodules and corresponding energy dispersive spectroscopy results (a-b: Microsphere structures and corresponding EDS results in the western Pacific nodules; c-d: Microsphere structures and corresponding EDS results in the eastern Pacific nodules.)
| [1] | 赵昌会, 叶德赞, 魏文铃. 深海微生物的研究进展[J]. 微生物学通报, 2006, 33(3):142-146. |
| ZHAO C H, YE D Z, WEI W L. Research on deep-sea microbiology[J]. Microbiology, 2006, 33(3): 142-146. | |
| [2] | 王风平, 周悦恒, 张新旭, 等. 深海微生物多样性[J]. 生物多样性, 2013, 21(4):446-456. |
| WANG F P, ZHOU Y H, ZHANG X X, et al. Biodiversity of deep-sea microorganisms[J]. Biodiversity Science, 2013, 21(4): 446-456. | |
| [3] | 井晓欢, 王杏, 熊尚凌, 等. 东太平洋多金属结核区两个站位深海沉积物细菌多样性[J]. 微生物学报, 2016, 56(9):1434-1449. |
| JING X H, WANG X, XIONG S L, et al. Bacterial diversity in deep-sea sediments from two stations in the east Pacific polymetallic nodule province[J]. Acta Microbiologica Sinica, 2016, 56(9): 1434-1449. | |
| [4] | PARKES R J, CRAGG B A, BALE S J, et al. Deep bacterial biosphere in Pacific Ocean sediments[J]. Nature, 1994, 371(6496): 410-413. |
| [5] | REYKHARD L Y, SHULGA N A. Fe-Mn nodule morphotypes from the NE Clarion-Clipperton Fracture zone, Pacific Ocean: Comparison of mineralogy, geochemistry and genesis[J]. Ore Geology Reviews, 2019, 110: 102933. |
| [6] | HEIN J R, KOSCHINSKY A, KUHN T. Deep-ocean poly-metallic nodules as a resource for critical materials[J]. Nature Reviews Earth & Environment, 2020, 1(3): 158-169. |
| [7] |
任江波, 邓义楠, 赖佩欣, 等. 太平洋调查区多金属结核的地球化学特征和成因[J]. 地学前缘, 2021, 28(2):412-425.
DOI |
|
REN J B, DENG Y N, LAI P X, et al. Geochemical characteristics and genesis of the polymetallic nodules in the Pacific survey area[J]. Earth Science Frontiers, 2021, 28(2): 412-425.
DOI |
|
| [8] | HALBACH P, SCHERHAG C, HEBISCH U, et al. Geochemical and mineralogical control of different genetic types of deep-sea nodules from the Pacific Ocean[J]. Mineralium Deposita, 1981, 16(1): 59-84. |
| [9] | JIANG X D, GONG J L, REN J B, et al. An interdependent relationship between microbial ecosystems and ferromanganese nodules from the Western Pacific Ocean[J]. Sedimentary Geology, 2020, 398: 105588. |
| [10] | 谢先德, 张刚生, 贾建业. 微生物-矿物相互作用之环境意义的研究[J]. 岩石矿物学杂志, 2001, 20(4):382-386. |
| XIE X D, ZHANG G S, JIA J Y. Environmental signifi-cance of the interaction between minerals and microbes[J]. Acta Petrologica et Mineralogica, 2001, 20(4): 382-386. | |
| [11] | 姜明玉, 胡艺豪, 于心科, 等. 大洋铁锰结核的微生物成矿过程及其研究进展[J]. 海洋科学, 2020, 44(7):156-164. |
| JIANG M Y, HU Y H, YU X K, et al. Advances in research on biological mineralization process of marine ferromanganese nodules[J]. Marine Sciences, 2020, 44(7): 156-164. | |
| [12] | HOFFMANN T D, REEKSTING B J, GEBHARD S. Bacteria-induced mineral precipitation: A mechanistic review[J]. Microbiology, 2021, 167(4): 001049. |
| [13] | 杜灵通, 吕新彪. 大洋多金属结核研究概况[J]. 地质与资源, 2003, 12(3):185-187. |
| DU L T, LÜ X B. A review of the study on polymetallic nodules in ocean[J]. Journal of Precious Metallic Geology, 2003, 12(3): 185-187. | |
| [14] | HEIN J R, MIZELL K, KOSCHINSKY A, et al. Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: Comparison with land-based resources[J]. Ore Geology Reviews, 2013, 51: 1-14. |
| [15] | XU M X, WANG P, WANG F P, et al. Microbial diversity at a deep-sea station of the Pacific nodule province[J]. Biodiversity & Conservation, 2005, 14: 3363-3380. |
| [16] |
ZHANG D C, LI X D, WU Y H, et al. Microbe-driven elemental cycling enables microbial adaptation to deep-sea ferromanganese nodule sediment fields[J]. Microbiome, 2023, 11(1): 160.
DOI PMID |
| [17] | 叶光斌, 王风平, 肖湘. 东太平洋中国多金属结核区锰结核样品中微生物群落结构的研究[J]. 台湾海峡, 2010, 29(2):218-227. |
| YE G B, WANG F P, XIAO X. Study of the microbial community structure of manganese nodule samples from China polymental nodule province in the Eastern Pacific Ocean[J]. Journal of Oceanography in Taiwan Strait, 2010, 29(2): 218-227. | |
| [18] | SHIRAISHI F, MITSUNOBU S, SUZUKI K, et al. Dense microbial community on a ferromanganese nodule from the ultra-oligotrophic South Pacific Gyre: Implications for biogeochemical cycles[J]. Earth and Planetary Science Letters, 2016, 447: 10-20. |
| [19] | NOVIKOV G V, MEL’NIKOV M E, BOGDANOVA O Y, et al. Nature of co-bearing ferromanganese crusts of the Magellan Seamounts (Pacific Ocean): Communication 1. Geology, mineralogy, and geochemistry[J]. Lithology and Mineral Resources, 2014, 49(1): 6197. |
| [20] | SHAO Q W, SUN D, FANG C, et al. Microbial food webs share similar biogeographic patterns and driving mechanisms with depths in oligotrophic tropical western Pacific Ocean[J]. Frontiers in Microbiology, 2023, 14: 1098264. |
| [21] | MACDONALD K C, FOX P J, ALEXANDER R T, et al. Volcanic growth faults and the origin of Pacific abyssal hills[J]. Nature, 1996, 380(6570): 125-129. |
| [22] | SINGER E, BUSHNELL B, COLEMAN-DERR D, et al. High-resolution phylogenetic microbial community profiling[J]. The ISME Journal, 2016, 10(8): 2020-2032. |
| [23] |
EDGAR R C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads[J]. Nature Methods, 2013, 10: 996-998.
DOI PMID |
| [24] |
QUAST C, PRUESSE E, YILMAZ P, et al. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools[J]. Nucleic Acids Research, 2013, 41: 590-596.
DOI PMID |
| [25] | YANG K H, DONG Y H, LI Z G, et al. Geochemistry of buried polymetallic nodules from the eastern Pacific Ocean: Implication for the depth-controlled alteration process[J]. Marine Geology, 2024, 467: 107190. |
| [26] | JIANG X D, ZHAO X, CHOU Y M, et al. Characterization and quantification of magnetofossils within abyssal manganese nodules from the western Pacific Ocean and implications for nodule formation[J]. Geochemistry, Geophysics, Geosystems, 2020, 21(3): e2019GC008811. |
| [27] | BLÖTHE M, WEGORZEWSKI A, MÜLLER C, et al. Manganese-cycling microbial communities inside deep-sea manganese nodules[J]. Environmental Science & Technology, 2015, 49(13): 7692-7700. |
| [28] | SHULSE C N, MAILLOT B, SMITH C R, et al. Polymetallic nodules, sediments, and deep waters in the equatorial North Pacific exhibit highly diverse and distinct bacterial, archaeal, and microeukaryotic communities[J]. Microbiology Open, 2017, 6(2): e00428. |
| [29] | NEETHU C S, SARAVANAKUMAR C, PURVAJA R, et al. Arsenic resistance and horizontal gene transfer are associated with carbon and nitrogen enrichment in bacteria[J]. Environmental Pollution, 2022, 311: 119937. |
| [30] |
BEN SALEM F, BEN SAID O, CRAVO-LAUREAU C, et al. Bacterial community assemblages in sediments under high anthropogenic pressure at Ichkeul Lake/Bizerte Lagoon hydrological system, Tunisia[J]. Environmental Pollution, 2019, 252: 644-656.
DOI PMID |
| [31] | WEI X, OUYANG K H, LONG T H, et al. Dynamic variations in rumen fermentation characteristics and bacterial community composition during in vitro fermentation[J]. Fermentation, 2022, 8(6): 276. |
| [32] | MCKEE L S, LA ROSA S L, WESTERENG B, et al. Polysaccharide degradation by the bacteroidetes: Mechanisms and nomenclature[J]. Environmental Microbiology Reports, 2021, 13(5): 559-581. |
| [33] | MUNOZ R, TEELING H, AMANN R, et al. Ancestry and adaptive radiation of Bacteroidetes as assessed by comparative genomics[J]. Systematic and Applied Microbiology, 2020, 43(2): 126065. |
| [34] |
KLIMEK D, HEROLD M, CALUSINSKA M. Comparative genomic analysis of Planctomycetota potential for polysaccharide degradation identifies biotechnologically relevant microbes[J]. BMC Genomics, 2024, 25(1): 523.
DOI PMID |
| [35] | BARKA E A, VATSA P, SANCHEZ L, et al. Taxonomy, physiology, and natural products of Actinobacteria[J]. Microbiology and Molecular Biology Reviews, 2015, 80(1): 1-43. |
| [36] | RANJANI A, DHANASEKARAN D, GOPINATH P M. An introduction to Actinobacteria[M] //DHANASEKARAN D, JIANGY. Actinobacteria-basics and biotechnological applications. [S.l.]: InTech, 2016. |
| [37] | ABRAHAM W R, MACEDO A J, LÜNSDORF H, et al. Arcicella[M]//Bergey’s manual of systematics of archaea and bacteria. Hoboken, NJ: Wiley, 2015: 1-5. |
| [38] | CHEN W M, YANG S H, YOUNG C C, et al. Arcicella rigui sp. nov., isolated from water of a wetland, and emended descriptions of the genus Arcicella, Arcicella aquatica, Arcicella rosea and Arcicella aurantiaca[J]. International Journal of Systematic and Evolutionary Microbiology, 2013, 63: 134-140. |
| [39] | BACOSA H P, ERDNER D L, ROSENHEIM B E, et al. Hydrocarbon degradation and response of seafloor sediment bacterial community in the northern Gulf of Mexico to light Louisiana sweet crude oil[J]. The ISME Journal, 2018, 12(10): 2532-2543. |
| [40] |
TULLY B J, HEIDELBERG J F. Microbial communities associated with ferromanganese nodules and the surrounding sediments[J]. Frontiers in Microbiology, 2013, 4: 161.
DOI PMID |
| [41] | WU Y H, LIAO L, WANG C S, et al. A comparison of microbial communities in deep-sea polymetallic nodules and the surrounding sediments in the Pacific Ocean[J]. Deep Sea Research Part I: Oceanographic Research Papers, 2013, 79: 40-49. |
| [42] | MOLARI M, JANSSEN F, VONNAHME T R, et al. The contribution of microbial communities in polymetallic nodules to the diversity of the deep-sea microbiome of the Peru Basin (4130-4198 m depth)[J]. Biogeosciences, 2020, 17(12): 3203-3222. |
| [43] | ZHAO X, LIU B F, WANG X H, et al. Single molecule sequencing reveals response of manganese-oxidizing microbiome to different biofilter media in drinking water systems[J]. Water Research, 2020, 171: 115424. |
| [44] | GARCÍA M T, MELLADO E, OSTOS J C, et al. Halomonas organivorans sp. nov., a moderate halophile able to degrade aromatic compounds[J]. International Journal of Systematic and Evolutionary Microbiology, 2004, 54: 1723-1728. |
| [45] | QIU X, YU L B, CAO X R, et al. Halomonas sedimenti sp. nov., a halotolerant bacterium isolated from deep-sea sediment of the southwest Indian Ocean[J]. Current Microbiology, 2021, 78(4): 1662-1669. |
| [46] |
NOIRUNGSEE N, HACKBUSCH S, VIAMONTE J, et al. Influence of oil, dispersant, and pressure on microbial communities from the Gulf of Mexico[J]. Scientific Reports, 2020, 10(1): 7079.
DOI PMID |
| [47] | SCHNEIKER S, DOS SANTOS V A P M, BARTELS D, et al. Genome sequence of the ubiquitous hydrocarbon-degrading marine bacterium Alcanivorax borkumensis[J]. Nature Biotechnology, 2006, 24(8): 997-1004. |
| [48] | ETTWIG K F, BUTLER M K, LE PASLIER D, et al. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria[J]. Nature, 2010, 464(7288): 543-548. |
| [49] |
LAURO F M, MCDOUGALD D, THOMAS T, et al. The genomic basis of trophic strategy in marine bacteria[J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(37): 15527-15533.
DOI PMID |
| [50] | CORAM N J, RAWLINGS D E. Molecular relationship between two groups of the genus Leptospirillum and the finding that Leptospirillum ferriphilum sp. nov. dominates South African commercial biooxidation tanks that operate at 40 degrees C[J]. Applied and Environmental Microbiology, 2002, 68(2): 838-845. |
| [51] | SWEETMAN A K, SMITH C R, SHULSE C N, et al. Key role of bacteria in the short-term cycling of carbon at the abyssal seafloor in a low particulate organic carbon flux region of the eastern Pacific Ocean[J]. Limnology and Oceanography, 2019, 64(2): 694-713. |
| [52] | ZHANG L, HUANG X Y, ZHOU J Z, et al. Active predation, phylogenetic diversity, and global prevalence of myxobacteria in wastewater treatment plants[J]. The ISME Journal, 2023, 17(5): 671-681. |
| [53] |
SHULGA N, ABRAMOV S, KLYUKINA A, et al. Fast-growing Arctic Fe-Mn deposits from the Kara Sea as the refuges for cosmopolitan marine microorganisms[J]. Scientific Reports, 2022, 12(1): 21967.
DOI PMID |
| [54] | SUN X, KOP L F M, LAU M C Y, et al. Uncultured Nitrospina-like species are major nitrite oxidizing bacteria in oxygen minimum zones[J]. The ISME Journal, 2019, 13(10): 2391-2402. |
| [55] | GAO Y H, WU J J, ZHANG D, et al. The impact of alloying element Cu on corrosion and biofilms of 316L stainless steel exposed to seawater[J]. Environmental Science and Pollution Research International, 2024, 31(12): 18842-18855. |
| [56] | ESPINOSA E, MARCO-NOALES E, GÓMEZ D, et al. Taxonomic study of Marinomonas strains isolated from the seagrass Posidonia oceanica, with descriptions of Marinomonas balearica sp. nov. and Marinomonas pollencensis sp. nov[J]. International Journal of Systematic and Evolutionary Microbiology, 2010, 60: 93-98. |
| [57] |
BOEUF D, EDWARDS B R, EPPLEY J M, et al. Biological composition and microbial dynamics of sinking particulate organic matter at abyssal depths in the oligotrophic open ocean[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(24): 11824-11832.
DOI PMID |
| [58] | HOLLINGSWORTH A L, JONES D O B, YOUNG C R. Spatial variability of abyssal nitrifying microbes in the north-eastern Clarion-Clipperton Zone[J]. Frontiers in Marine Science, 2021, 8: 663420. |
| [59] |
ZHANG L F, FU G K, ZHANG Z. Simultaneous nutrient and carbon removal and electricity generation in self-buffered biocathode microbial fuel cell for high-salinity mustard tuber wastewater treatment[J]. Bioresource Technology, 2019, 272: 105-113.
DOI PMID |
| [60] | SOROKIN D Y, RAINEY F A, WEBB R I, et al. Sulfitobacter[M]//Bergey’s manual of systematics of archaea and bacteria. Hoboken, NJ: Wiley, 1996: 1-8. |
| [61] |
SWAN B K, MARTINEZ-GARCIA M, PRESTON C M, et al. Potential for chemolithoautotrophy among ubiquitous bacteria lineages in the dark ocean[J]. Science, 2011, 333(6047): 1296-1300.
DOI PMID |
| [62] | QIU D R, WEI H H, TU Q C, et al. Combined genomics and experimental analyses of respiratory characteristics of Shewanella putrefaciens W3-18-1[J]. Applied and Environ-mental Microbiology, 2013, 79(17): 5250-5257. |
| [63] | VANDIEKEN V, PESTER M, FINKE N, et al. Three manganese oxide-rich marine sediments harbor similar commu-nities of acetate-oxidizing manganese-reducing bacteria[J]. The ISME Journal, 2012, 6(11): 2078-2090. |
| [64] | LÜ Q, ZHANG B G, XING X, et al. Biosynthesis of copper nanoparticles using Shewanella loihica PV-4 with antibacterial activity: Novel approach and mechanisms investigation[J]. Journal of Hazardous Materials, 2018, 347: 141-149. |
| [65] | BRÄUER S L, ADAMS C, KRANZLER K, et al. Culturable Rhodobacter and Shewanella species are abundant in estuarine turbidity maxima of the Columbia River[J]. Environmental Microbiology, 2011, 13(3): 589-603. |
| [66] | MATSUNAGA T, SAKAGUCHI T, TADAKORO F. Magnetite formation by a magnetic bacterium capable of growing aerobically[J]. Applied Microbiology and Biotechnology, 1991, 35(5): 651-655. |
| [67] | CHOI A, YANG S J, RHEE K H, et al. Lentisphaera marina sp. nov., and emended description of the genus Lentisphaera[J]. International Journal of Systematic and Evolutionary Microbiology, 2013, 63: 1540-1544. |
| [68] | TEMPLETON A S, STAUDIGEL H, TEBO B M. Diverse Mn(II)-oxidizing bacteria isolated from submarine basalts at Loihi seamount[J]. Geomicrobiology Journal, 2005, 22(3/4): 127-139. |
| [69] | HANDLEY K M, LLOYD J R. Biogeochemical implications of the ubiquitous colonization of marine habitats and redox gradients by Marinobacter species[J]. Frontiers in Microbiology, 2013, 4: 136. |
| [70] | LIN X Z, GAO A G, CHEN H W. Isolation and phylogenetic analysis of cultivable manganese bacteria in sediments from the Arctic Ocean[J]. Acta Ecologica Sinica, 2008, 28(12): 6364-6370. |
| [71] | TEBO B M, BARGAR J R, CLEMENT B G, et al. Biogenic manganese oxides: Properties and mechanisms of formation[J]. Annual Review of Earth and Planetary Sciences, 2004, 32: 287-328. |
| [72] | ANDERSON C R, JOHNSON H A, CAPUTO N, et al. Mn(II) oxidation is catalyzed by heme peroxidases in “Aurantimonas manganoxydans” strain SI85-9A1 and Erythrobacter sp. strain SD-21[J]. Applied and Environ-mental Microbiology, 2009, 75(12): 4130-4138. |
| [73] | DICK G J, TORPEY J W, BEVERIDGE T J, et al. Direct identification of a bacterial manganese(II) oxidase, the multicopper oxidase MnxG, from spores of several different marine Bacillus species[J]. Applied and Environmental Microbiology, 2008, 74(5): 1527-1534. |
| [74] | BROUWERS G J, VIJGENBOOM E, CORSTJENS P L A M, et al. Bacterial Mn2+oxidizing systems and multicopper oxidases: An overview of mechanisms and functions[J]. Geomicrobiology Journal, 2000, 17(1): 1-24. |
| [75] |
WOLFAARDT G M, LAWRENCE J R, ROBARTS R D, et al. Bioaccumulation of the herbicide diclofop in extracellular polymers and its utilization by a biofilm community during starvation[J]. Applied and Environmental Microbiology, 1995, 61(1): 152-158.
DOI PMID |
| [76] |
WANG X H, MÜLLER W E G. Marine biominerals: Perspectives and challenges for polymetallic nodules and crusts[J]. Trends in Biotechnology, 2009, 27(6): 375-383.
DOI PMID |
| [1] | 谷树韬, 连涛. 使用再分析资料检测西北太平洋热带气旋的一种新型追踪器[J]. 海洋学研究, 2024, 42(4): 34-42. |
| [2] | 罗通, 洪加诚. 基于机器学习的热带气旋快速增强预报[J]. 海洋学研究, 2024, 42(3): 99-107. |
| [3] | 王添翼, 董彦辉, 初凤友, 石学法, 李小虎, 苏蓉, 章伟艳. 太平洋深海富稀土沉积物的分类及成因[J]. 海洋学研究, 2024, 42(1): 23-35. |
| [4] | 张旭东, 丘仲锋, 毛科峰, 王鹏皓. 西北太平洋中尺度涡合成结构及其对声传播的影响[J]. 海洋学研究, 2024, 42(1): 58-68. |
| [5] | 邬欣然, 董彦辉, 李正刚, 王浩, 章伟艳, 李怀明, 李小虎, 初凤友. 东太平洋CC区深海稀土资源潜力:沉积物地球化学标志[J]. 海洋学研究, 2023, 41(4): 46-56. |
| [6] | 孟宇, 陈双玲. 海水硝酸盐跃层深度计算方法研究[J]. 海洋学研究, 2023, 41(3): 1-13. |
| [7] | 龚芳, 朱伯仲, 李腾, 王雨馨, 李鸿喆, 何贤强, 张清. 南太平洋典型岛国海洋生态环境状况及其对汤加火山爆发的响应[J]. 海洋学研究, 2023, 41(3): 101-114. |
| [8] | 邓韬, 许冬, 肖婷露, 叶黎明, 章伟艳. 西太平洋海山盆地沉积物黏土矿物特征及其指示意义[J]. 海洋学研究, 2023, 41(3): 56-72. |
| [9] | 祝飞扬, 李怀明, 姚鹏飞, 王潇, 朱继浩, 吕士辉, 罗祎, 周丽娜, 刘禹维, 唐煜童. 两相淋滤实验在深海铁锰结核研究中的应用[J]. 海洋学研究, 2023, 41(2): 83-93. |
| [10] | 蒋佳茗, 汪亦蕾. 热带西北太平洋0~300 m热含量的年代际变化[J]. 海洋学研究, 2022, 40(1): 1-11. |
| [11] | 许钰佳, 陈长霖, 彭旭东, 刘磊, . 西北太平洋热带气旋路径预报偏差分析[J]. 海洋学研究, 2021, 39(2): 1-11. |
| [12] | 李月, 许冬, 张志毅, 蒋科迪, 刘庚. 西太平洋E20岩芯末次冰期以来的沉积特征与环境意义[J]. 海洋学研究, 2021, 39(2): 12-20. |
| [13] | 王苑如, 崔鸿鹏, 李继东, 孙栋, 王春生, 杨娟. 西太平洋多金属结核区表层沉积物细菌群落结构及其对沉积扰动的响应[J]. 海洋学研究, 2021, 39(2): 21-32. |
| [14] | 刘瀚仁, 廖一波, 寿鹿, 曾江宁, 汤雁滨, 刘清河, 谭勇华, 吕兑安, 程杰. 浙江苍南无居民海岛岩相潮间带生物群落多样性研究[J]. 海洋学研究, 2021, 39(2): 68-79. |
| [15] | 贾海波, 柴小平, 黄备. 2016—2019年长江口海域季节性低氧对大型底栖动物群落的影响[J]. 海洋学研究, 2021, 39(2): 80-88. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||